🔥 What Nuclear Fusion Really Means for MCAT Students
Nuclear fusion is more than an astrophysics concept it’s a recurring MCAT topic that connects mass energy conversion, nuclear stability, and isotope behavior. The reaction between deuterium and tritium is the classic example used in exam passages, and understanding this single process helps decode multiple high-yield physics and chemistry questions.
⚛️ Breaking Down the KOTC Fusion Visual
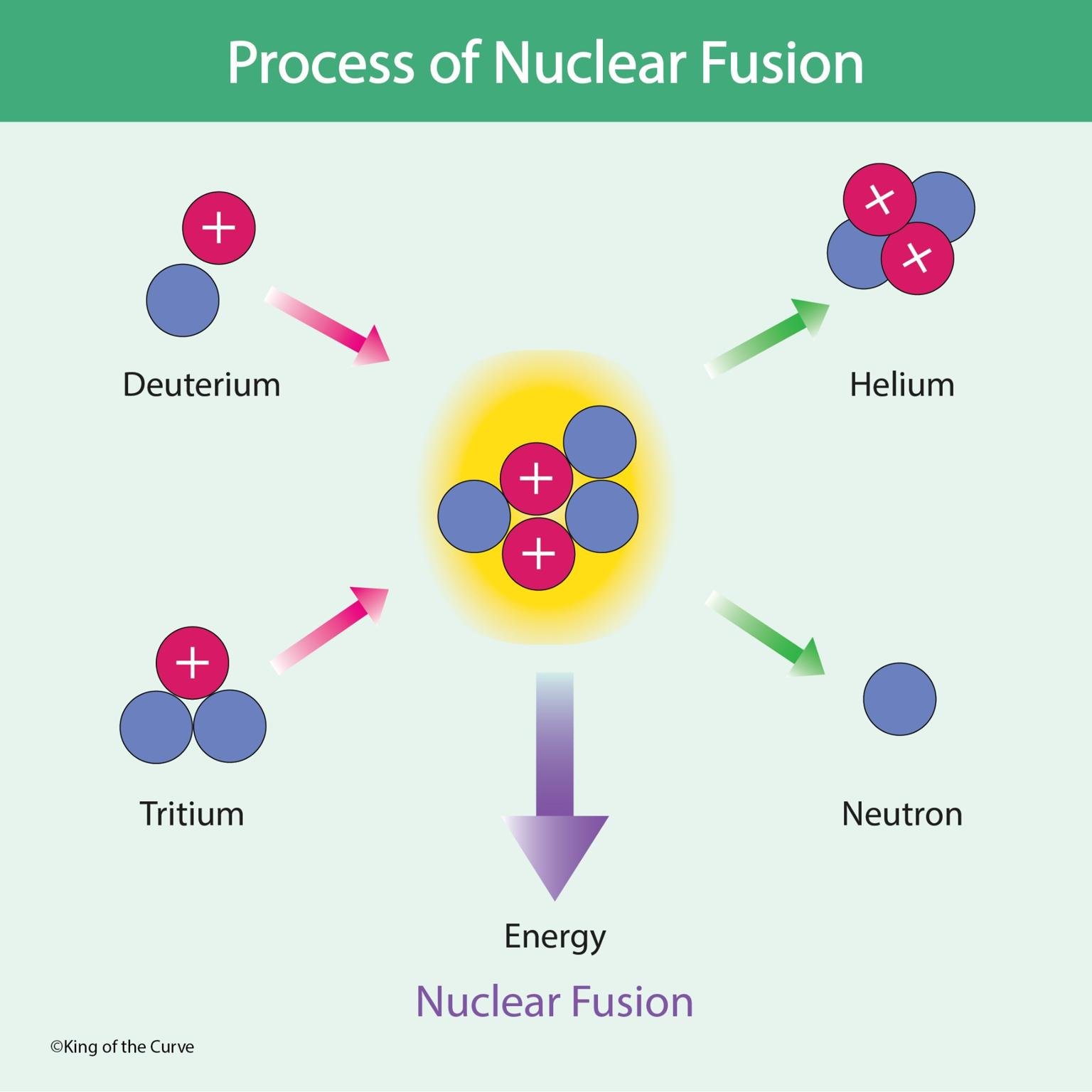
In the King of the Curve fusion illustration, deuterium (one proton, one neutron) and tritium (one proton, two neutrons) collide with enough energy to overcome electrostatic repulsion. When they fuse, the resulting helium-4 nucleus is far more stable, and this increased stability drives the release of a neutron and a significant amount of energy.
🌟 Why Fusion Releases So Much Energy
Fusion produces a nucleus with higher binding energy per nucleon, meaning the products are more stable than the reactants. This increased stability results in a mass defect—lost mass—which is converted to energy using Einstein’s equation E = mc². MCAT questions use this concept to assess your understanding of nuclear stability and energy relationships.
📘 High-Yield MCAT Connections
Fusion questions typically assess conservation of mass number, conservation of charge, qualitative reasoning about nuclear forces, and isotope identification. Passages may use stellar environments or reactor-style contexts, but the underlying skills tested are simple: track particles, balance reactions, and understand why stability influences energy release.
📊 Quick Reference Table: Key Fusion Components
| Particle / Product | Composition | MCAT Relevance |
|---|---|---|
| Deuterium (²H) | 1 proton, 1 neutron | Introduces isotope notation and nuclear stability |
| Tritium (³H) | 1 proton, 2 neutrons | Connects to radioactivity and decay patterns |
| Helium-4 | 2 protons, 2 neutrons | Demonstrates stability and binding energy concepts |
| Neutron | 1 neutron | Common product in nuclear reactions; may drive secondary processes |
| Energy | Mass defect → E = mc² | Tests conceptual or calculation-based physics |
🚀 How This One Concept Can Boost Your Score
Mastering the deuterium–tritium fusion example prepares you for MCAT questions involving isotopes, conservation rules, binding energy trends, and mass–energy conversion. These appear across physics, chemistry, and data-interpretation sections, making fusion one of the most transferable nuclear topics to study.
📚 Strengthening Your Understanding With KOTC Tools
Reinforcing this concept through King of the Curve’s Adaptive Q-Bank, daily questions, and science refreshers at kingofthecurve.org/studyscience promotes long-term retention and exam-ready confidence. Fusion may power the Sun, but paired with the right tools, it can also power a stronger MCAT performance.
Frequently Asked Questions (FAQs)
-
Aim for 4-6 focused hours, ensuring you incorporate breaks to avoid burnout.
-
Practice mindfulness techniques, take practice exams under realistic conditions, and maintain a balanced lifestyle.
-
Set short-term goals, seek support from mentors, and reward yourself for small achievements.
-
Regular exercise improves focus, reduces stress, and enhances overall mental clarity.
-
KOTC offers personalized learning tools, gamification features, and adaptive question banks to help students stay on track without burnout.