🧪 Solution Dilution: Understanding the Concept
Solution dilution is a fundamental concept in chemistry and biology that explains how the concentration of a solution changes when solvent is added. Importantly, dilution does not change the amount of solute, only the total volume of the solution.
🔬 What Is Dilution?
Dilution is the process of reducing the concentration of a solute in a solution by adding more solvent. As understanding dilution is essential in laboratories, medicine, and research, it forms the basis of accurate solution preparation.
⚗️ Before Dilution: Initial State
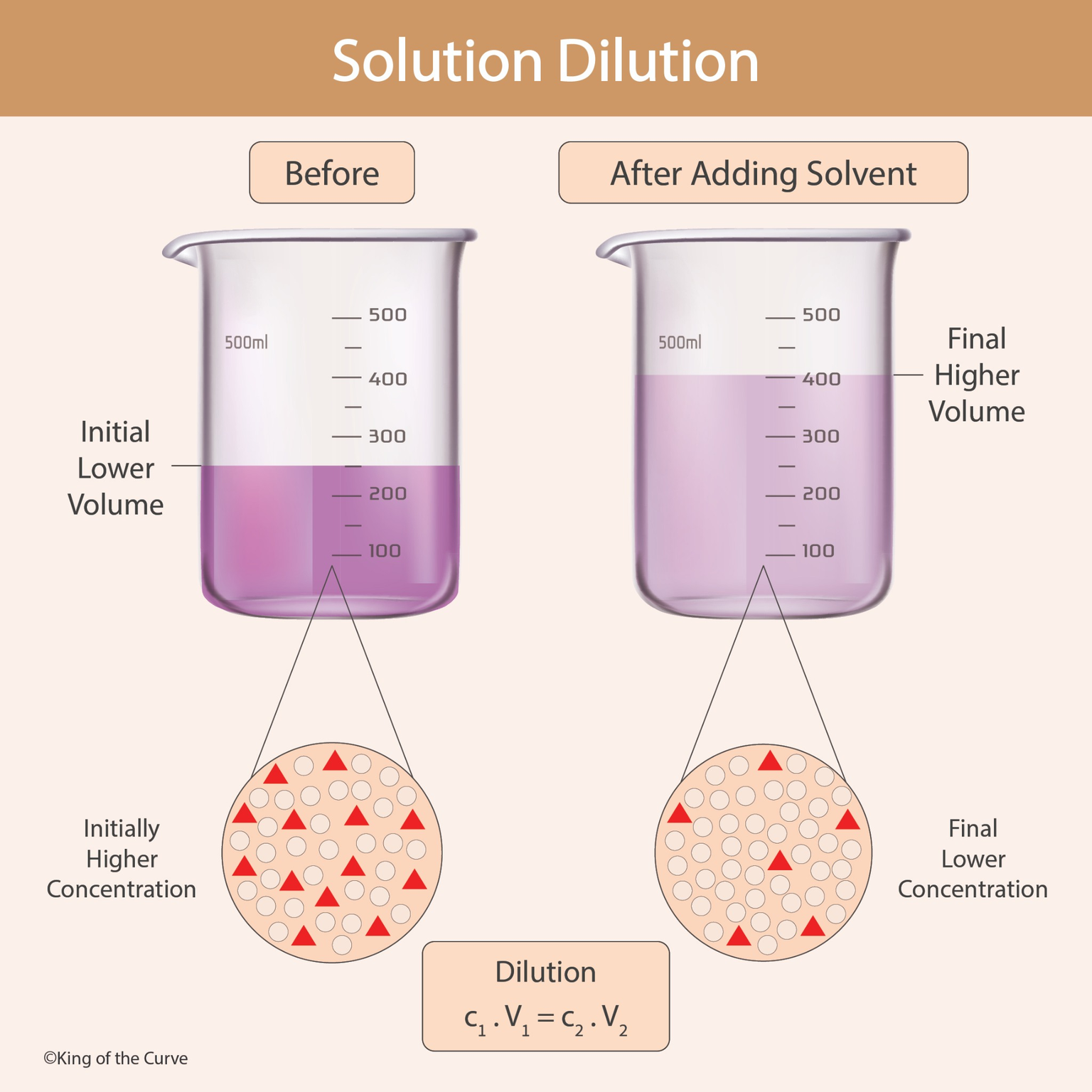
Before dilution:
The solution has a lower volume
The solute amount is fixed
Solute particles are closely packed
This results in a higher concentration
💧 After Adding Solvent: Diluted State
After dilution:
The volume increases
The solute amount remains unchanged
Solute particles become more spread out
The solution now has a lower concentration
📊 Comparison Table: Before vs After Dilution
| Feature | Before Dilution | After Dilution |
|---|---|---|
| Solution Volume | Lower | Higher |
| Amount of Solute | Constant | Constant |
| Solute Particle Density | High | Low |
| Concentration | High | Low |
| Appearance | Darker / More intense | Lighter / Less intense |
📐 The Dilution Formula
The dilution process follows this fundamental equation:
C₁ × V₁ = C₂ × V₂
Where:
C₁ = initial concentration
V₁ = initial volume
C₂ = final concentration
V₂ = final volume
This equation works because the total amount of solute remains unchanged during dilution.
🧠 Why Dilution Is Important
Dilution is widely used in:
Laboratory solution preparation
Drug dosing and IV fluids
Microbiology and serial dilutions
Biochemical and diagnostic testing
✅ Key Takeaway
Dilution increases the volume of a solution while decreasing its concentration, without altering the amount of solute. Understanding dilution and its formula is essential for accurate scientific and medical practice.
Frequently Asked Questions (FAQs)
-
Aim for 4-6 focused hours, ensuring you incorporate breaks to avoid burnout.
-
Practice mindfulness techniques, take practice exams under realistic conditions, and maintain a balanced lifestyle.
-
Set short-term goals, seek support from mentors, and reward yourself for small achievements.
-
Regular exercise improves focus, reduces stress, and enhances overall mental clarity.
-
KOTC offers personalized learning tools, gamification features, and adaptive question banks to help students stay on track without burnout.