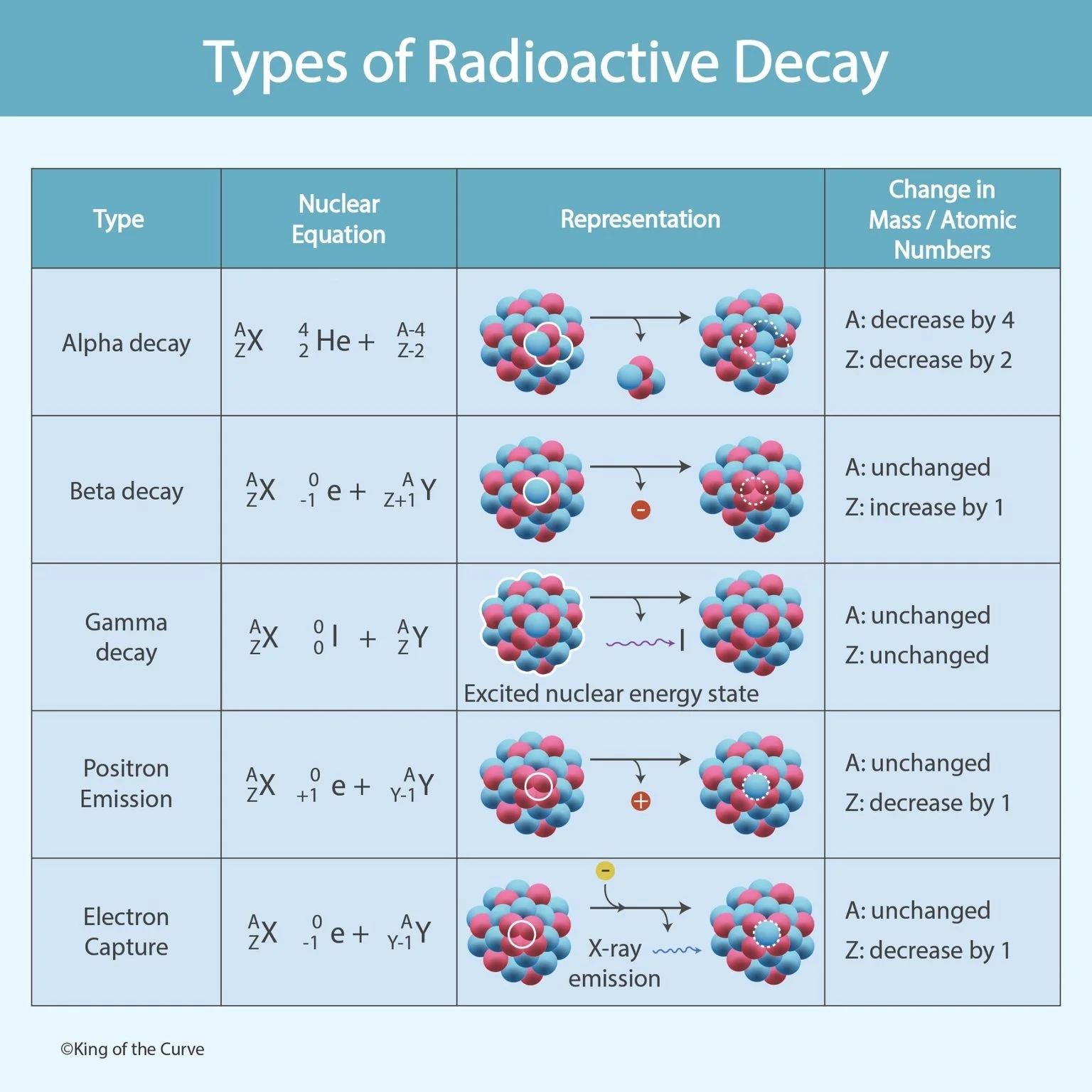
📘 Types of Radioactive Decay
Radioactive decay is a natural process where an unstable atomic nucleus releases energy to become more stable. This happens because some atoms contain an imbalance of protons and neutrons, and nature “fixes” this imbalance through the emission of particles or radiation. The result is a transformation of the atom into a more stable form sometimes even into a completely different element.
There are several types of radioactive decay, and each one affects the mass number (A) and atomic number (Z) in different ways. Understanding these changes is essential in physics, chemistry, medicine, nuclear energy, and even archaeology (like carbon dating). The chart above summarizes the major decay types and shows how the nucleus changes after each process.
⚛️ 1. Alpha (α) Decay
Alpha decay occurs when a heavy nucleus ejects an alpha particle, which is basically a helium nucleus (2 protons + 2 neutrons). Because it loses 4 nucleons total, the mass number decreases by 4, and the atomic number decreases by 2. Alpha decay is common in heavy elements like uranium and radon. Alpha particles are highly ionizing but do not travel far—they can be blocked by paper or skin.
⚡ 2. Beta (β⁻) Decay
In beta decay, a neutron inside the nucleus converts into a proton and releases an electron (β particle). This means the mass number stays the same, but the atomic number increases by 1 because the nucleus now has one extra proton. Beta decay happens when a nucleus has too many neutrons and tries to become more stable by balancing the proton-to-neutron ratio. Beta particles can travel farther than alpha particles and require thin metal or plastic shielding.
🌟 3. Gamma (γ) Decay
Gamma decay happens when the nucleus releases excess energy in the form of gamma radiation, which is a high-energy electromagnetic wave (not a particle). This decay doesn’t change the mass number or atomic number at all—it simply lowers the nucleus from an excited energy state to a stable one. Gamma rays are extremely penetrating and require thick materials like lead or concrete to block them. Gamma emission often occurs after alpha or beta decay.
➕ 4. Positron Emission (β⁺ Decay)
Positron emission occurs when a proton converts into a neutron and releases a positron, which is the antimatter version of an electron. Since one proton becomes a neutron, the atomic number decreases by 1, but the mass number remains unchanged. This decay happens when a nucleus has too many protons. Positron emission is important in medicine—especially in PET scans, where positrons collide with electrons and produce detectable gamma rays.
🧲 5. Electron Capture
Electron capture happens when the nucleus captures an inner-shell electron and combines it with a proton to form a neutron. Like positron emission, the atomic number decreases by 1 and mass number remains unchanged. This process often produces X-ray emission due to electron rearrangement in atomic shells after the capture. Electron capture is another method used by proton-heavy nuclei to achieve stability.
📊 Summary Table: How Each Decay Changes A and Z
| Decay Type | What is Emitted? | Mass Number (A) | Atomic Number (Z) |
|---|---|---|---|
| Alpha (α) | Helium nucleus (²⁴He) | Decreases by 4 | Decreases by 2 |
| Beta (β⁻) | Electron (⁰₋₁e) | Unchanged | Increases by 1 |
| Gamma (γ) | Energy (radiation) | Unchanged | Unchanged |
| Positron (β⁺) | Positron (⁰₊₁e) | Unchanged | Decreases by 1 |
| Electron Capture | Captured electron | Unchanged | Decreases by 1 |
✅ Why Learning These Decay Types Matters
Radioactive decay is not just a classroom topic—it impacts real-world science every day. Nuclear reactors rely on decay chains for energy production. Medical imaging uses gamma rays and positron emission. Cancer treatments use targeted radiation. Even fossil dating depends on understanding decay rates. Once you understand how A and Z change in each decay process, you can predict what element forms after decay and interpret nuclear equations with confidence.
Frequently Asked Questions (FAQs)
-
Aim for 4-6 focused hours, ensuring you incorporate breaks to avoid burnout.
-
Practice mindfulness techniques, take practice exams under realistic conditions, and maintain a balanced lifestyle.
-
Set short-term goals, seek support from mentors, and reward yourself for small achievements.
-
Regular exercise improves focus, reduces stress, and enhances overall mental clarity.
-
KOTC offers personalized learning tools, gamification features, and adaptive question banks to help students stay on track without burnout.