⚛️ Mass Defect: The Mystery of Missing Atomic Mass
The concept of mass defect reveals one of the most fascinating truths in atomic physics — atoms don’t weigh exactly what we expect them to. When scientists calculate the mass of an atom by adding the weights of its individual protons, neutrons, and electrons, the result is slightly higher than the atom’s actual measured mass. This difference, called the mass defect, is not an error but a key to understanding the powerful forces within the atomic nucleus.
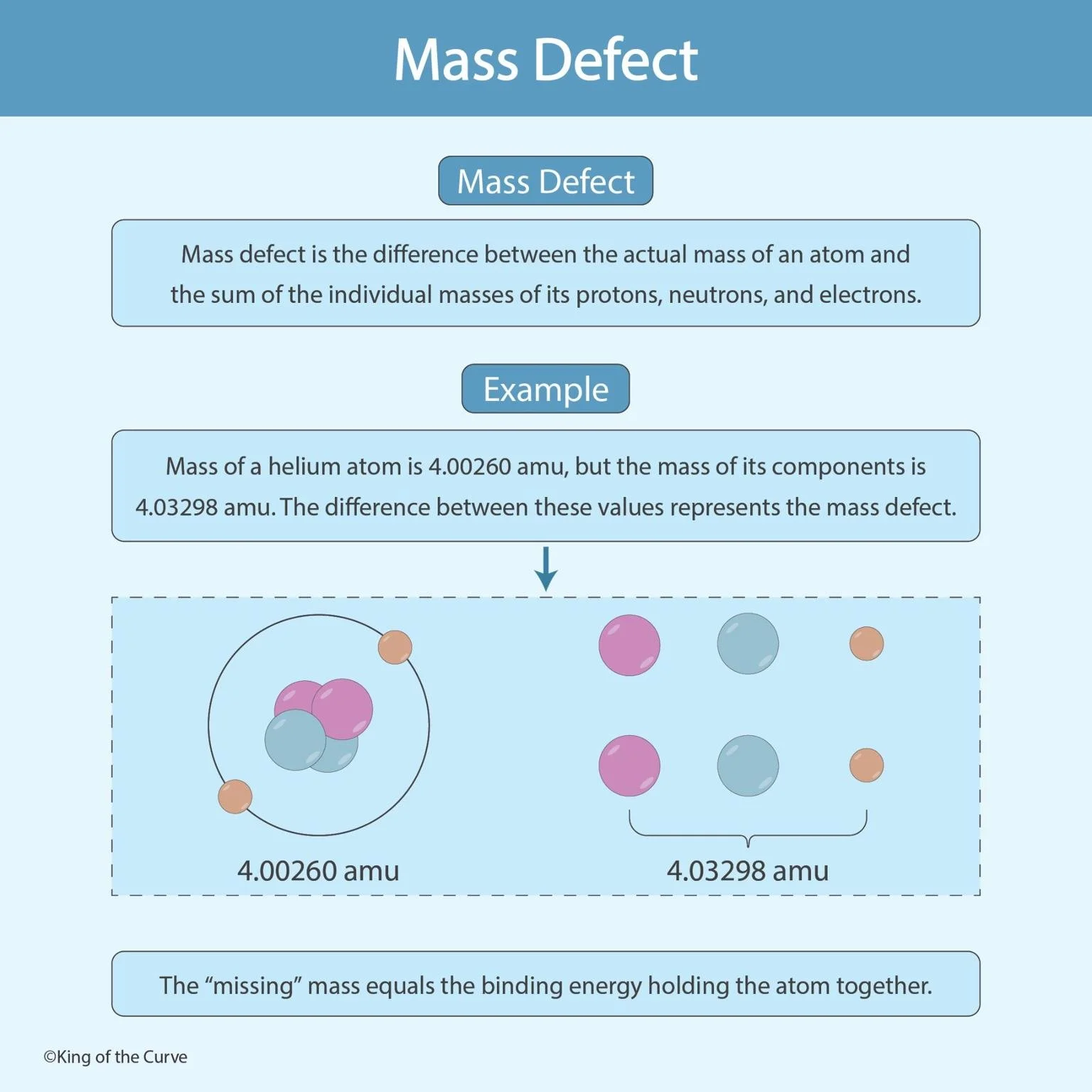
🧠 What Is the Mass Defect?
The mass defect is the difference between the actual mass of an atom and the total mass of its individual components. This missing mass is converted into binding energy, the energy that holds the nucleus together. According to Einstein’s famous equation, E = mc², even a small amount of missing mass corresponds to a large amount of energy.
When protons and neutrons bind together, some of their mass is transformed into energy. This “mass loss” doesn’t disappear — it becomes the binding energy that maintains nuclear stability. Without this conversion, atomic nuclei would not hold together, and matter as we know it couldn’t exist.
🔬 Example: The Helium Atom
Take the helium atom as an example. Its actual measured mass is 4.00260 amu, but when the masses of its individual protons, neutrons, and electrons are added, the total is 4.03298 amu. The difference of 0.03038 amu represents the mass defect — the portion of mass converted into binding energy that holds the helium nucleus together.
This small difference in mass corresponds to an enormous amount of energy. It’s this principle that powers stars, nuclear reactors, and even hydrogen bombs — demonstrating that the energy hidden within matter is far greater than what’s visible to us.
🌞 Why the Mass Defect Matters
Understanding mass defect is essential in explaining how nuclear energy is generated. In fusion reactions, such as those in the Sun, light nuclei combine to form heavier ones, releasing vast amounts of energy from small changes in mass. In fission, heavy nuclei split into lighter ones, again transforming mass into usable energy.
This concept also helps explain nuclear stability — atoms with a higher binding energy per nucleon are more stable, while those with lower values are more likely to undergo radioactive decay.
📊 Comparison: Mass Defect vs. Binding Energy
Below is a simple comparison to clarify how mass defect and binding energy relate to one another:
| ⚛️ Concept | 📘 Description | ⚡ Key Relationship |
|---|---|---|
| Mass Defect | The difference between the measured atomic mass and the total mass of its individual nucleons (protons and neutrons). | Represents the “missing” mass that has been converted into energy. |
| Binding Energy | The energy released when nucleons bind together to form a stable nucleus. | Calculated from the mass defect using Einstein’s equation E = mc². |
🚀 Final Thoughts
The mass defect showcases the elegant balance between matter and energy — a fundamental principle that powers the stars and fuels modern energy technology. It bridges the gap between physics and chemistry, showing how even the smallest changes in mass can lead to immense energy transformations.
🎯 Call to Action
Ready to deepen your understanding of physics and chemistry? Explore King of the Curve’s interactive learning tools — designed to simplify complex concepts like nuclear binding, atomic structure, and energy transformations. Learn visually, think critically, and ace your exams with science made simple! 🌟
Frequently Asked Questions (FAQs)
-
Aim for 4-6 focused hours, ensuring you incorporate breaks to avoid burnout.
-
Practice mindfulness techniques, take practice exams under realistic conditions, and maintain a balanced lifestyle.
-
Set short-term goals, seek support from mentors, and reward yourself for small achievements.
-
Regular exercise improves focus, reduces stress, and enhances overall mental clarity.
-
KOTC offers personalized learning tools, gamification features, and adaptive question banks to help students stay on track without burnout.