🔹 Coordinate Covalent Bonding: A Complete Guide
Coordinate covalent bonding is a special type of chemical bond where both electrons in the shared pair come from the same atom. This bonding plays an important role in many chemical reactions, especially in complex ion formation and molecular stability. Understanding coordinate covalent bonds helps students grasp how atoms can share electrons in different ways beyond normal covalent bonding.
⚛️ What Is a Coordinate Covalent Bond?
A coordinate covalent bond (also called a dative bond) is a covalent bond in which one atom provides both electrons for the shared pair. In ordinary covalent bonding, each atom contributes one electron. However, in coordinate bonding, one atom acts as the electron donor, while the other acts as the electron acceptor.
This bond is still considered covalent because the electrons are shared, but the source of the electron pair is unique.
🧪 Electron Donor and Electron Acceptor Atoms
Coordinate covalent bonding involves two key participants:
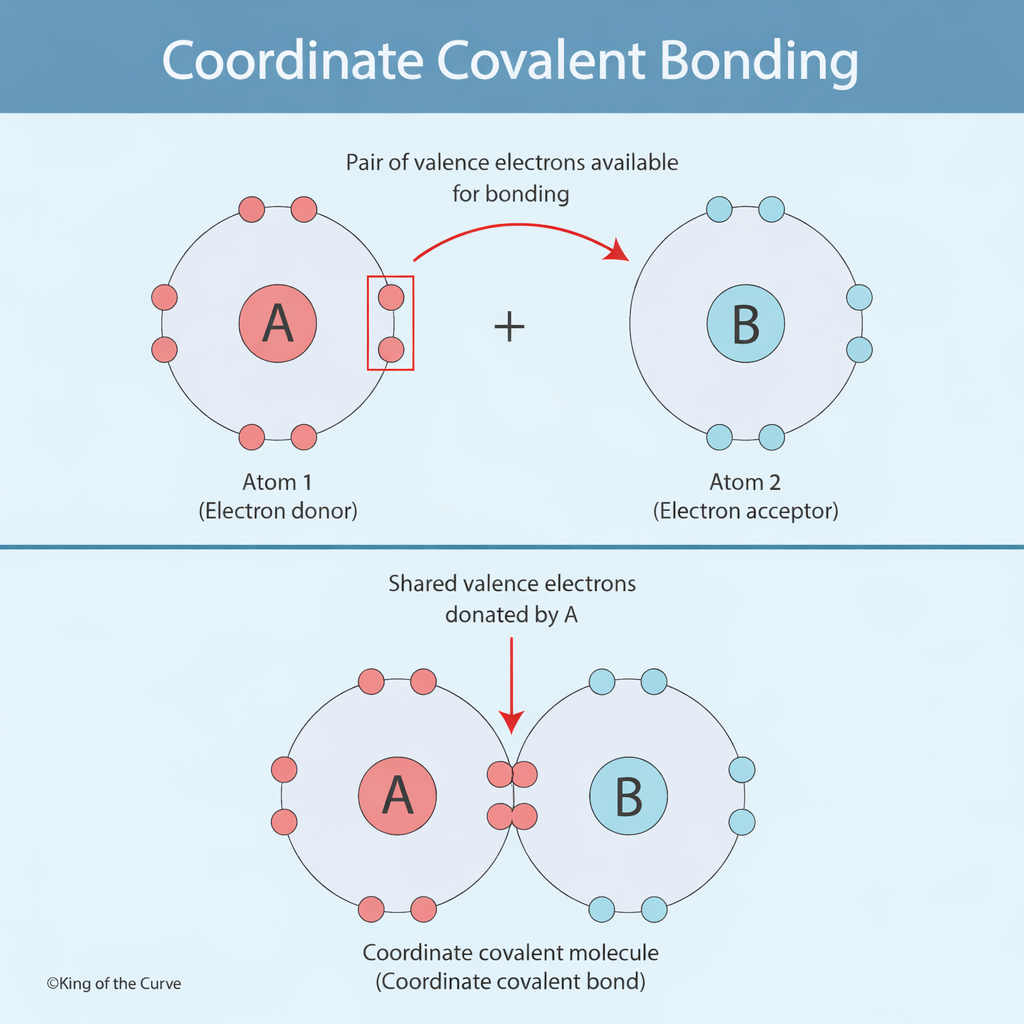
Atom 1 (Electron Donor): Has a lone pair of electrons available for bonding.
Atom 2 (Electron Acceptor): Has an empty orbital that can accept the electron pair.
In the infographic, atom A donates a pair of valence electrons, while atom B accepts them.
🔁 How Coordinate Covalent Bonds Form
The formation process occurs in steps:
Atom A contains a lone pair of electrons.
Atom B lacks electrons and has an available space for bonding.
Atom A donates both electrons to form a shared bond.
The result is a stable molecule held together by a coordinate covalent bond.
Once formed, the bond behaves like a normal covalent bond.
📌 Coordinate Covalent Bond Representation
Coordinate bonds are often shown using an arrow:
A → B
The arrow points from the donor atom to the acceptor atom, indicating the direction of electron donation.
🌍 Real-Life Examples of Coordinate Covalent Bonding
Coordinate covalent bonds are common in chemistry. Some well-known examples include:
Ammonium ion formation:
NH₃ + H⁺ → NH₄⁺Hydronium ion formation:
H₂O + H⁺ → H₃O⁺Complex ions:
Cu²⁺ + 4NH₃ → [Cu(NH₃)₄]²⁺
These bonds are essential in biological systems, coordination compounds, and acid-base reactions.
📊 Table: Coordinate Covalent Bond vs Regular Covalent Bond
| Feature | Regular Covalent Bond | Coordinate Covalent Bond |
|---|---|---|
| Electron Contribution | One electron from each atom | Both electrons from one atom |
| Donor–Acceptor Role | Not required | Required |
| Bond Type | Shared electron pair | Shared electron pair (donated) |
| Common Examples | H₂, Cl₂, CH₄ | NH₄⁺, H₃O⁺, metal complexes |
| Representation | Line (—) | Arrow (→) |
⭐ Importance of Coordinate Covalent Bonds
Coordinate covalent bonding is important because it explains:
Formation of ions in acids and bases
Stability of coordination compounds
Chemical bonding in complex molecules
Biological functions involving metal ions
It bridges the gap between simple covalent bonding and more advanced molecular interactions.
✅ Conclusion
Coordinate covalent bonding is a fascinating variation of covalent bonding where one atom supplies both bonding electrons. Although the bond forms differently, it functions like a standard covalent bond once established. Recognizing donor and acceptor atoms is key to understanding many chemical reactions and molecular structures.
Frequently Asked Questions (FAQs)
-
Aim for 4-6 focused hours, ensuring you incorporate breaks to avoid burnout.
-
Practice mindfulness techniques, take practice exams under realistic conditions, and maintain a balanced lifestyle.
-
Set short-term goals, seek support from mentors, and reward yourself for small achievements.
-
Regular exercise improves focus, reduces stress, and enhances overall mental clarity.
-
KOTC offers personalized learning tools, gamification features, and adaptive question banks to help students stay on track without burnout.