🧪 Orbitals on the Periodic Table: Understanding Electron Distribution
The periodic table is more than just an arrangement of elements; it’s a map that tells us where electrons live in atoms. These electron regions, called orbitals, define the chemical behavior of elements. Each orbital s, p, d, and f—represents a distinct shape and energy level that governs how electrons are organized within an atom.
⚛️ What Are Orbitals?
Orbitals are regions around the nucleus where electrons are most likely to be found. They do not follow fixed paths but instead exist in zones of probability. Each type of orbital (s, p, d, f) can hold a specific number of electrons:
s-orbital: 2 electrons
p-orbital: 6 electrons
d-orbital: 10 electrons
f-orbital: 14 electrons
This structured arrangement helps determine how atoms bond and interact, making orbitals the foundation of chemistry.
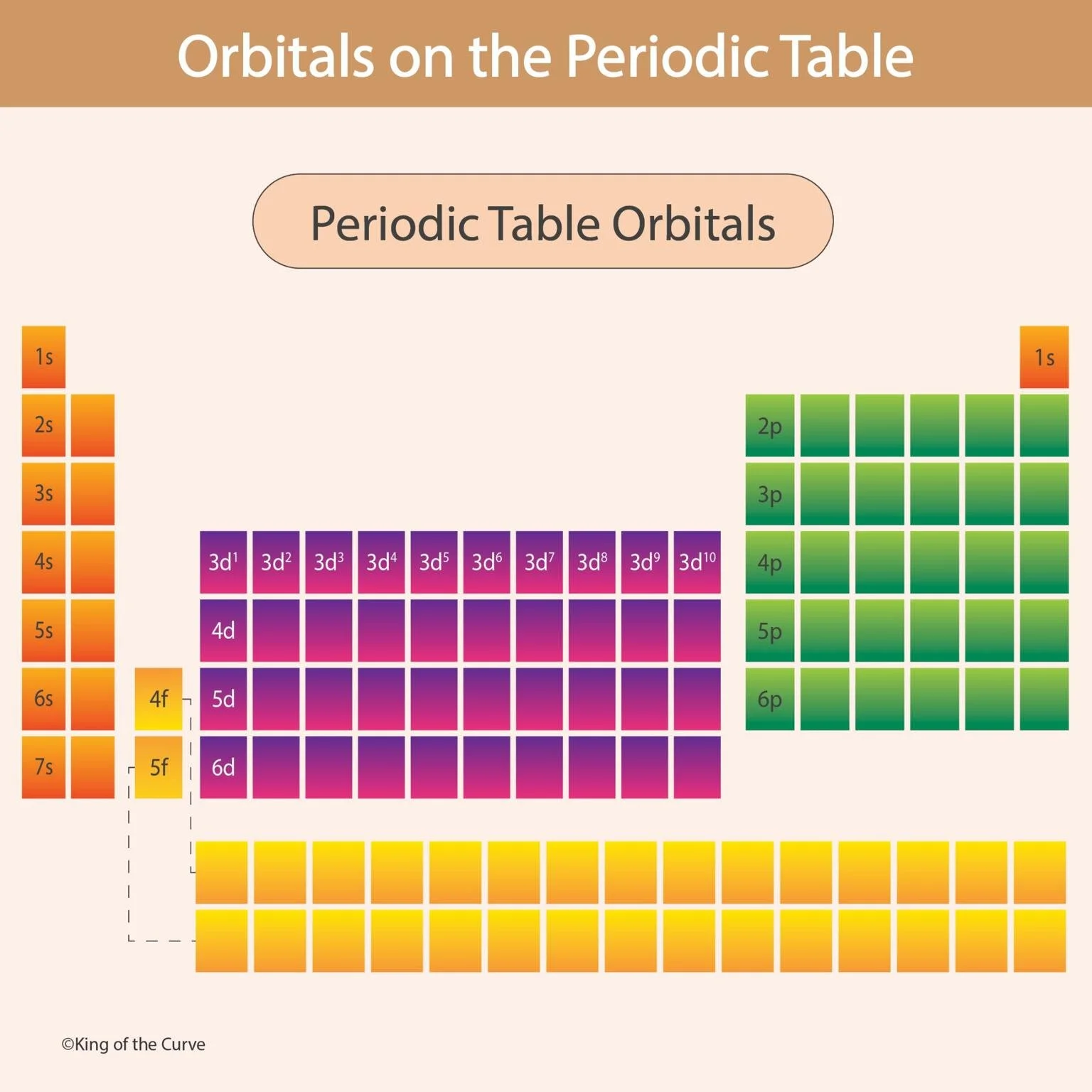
🌈 Arrangement of Orbitals on the Periodic Table
The periodic table is divided into four distinct blocks based on orbitals:
s-block: Groups 1 and 2 (alkali and alkaline earth metals)
p-block: Groups 13–18 (nonmetals, metalloids, and noble gases)
d-block: Transition metals in the center
f-block: Lanthanides and actinides (bottom two rows)
The progression of orbitals follows the Aufbau principle, filling from lower to higher energy levels (1s → 2s → 2p → 3s → 3p, and so on).
📊 Orbital Distribution Table
| Orbital Type | Maximum Electrons | Example Elements | Block on Periodic Table |
|---|---|---|---|
| s-orbital | 2 | Hydrogen (H), Magnesium (Mg) | s-block |
| p-orbital | 6 | Carbon (C), Oxygen (O), Chlorine (Cl) | p-block |
| d-orbital | 10 | Iron (Fe), Copper (Cu), Zinc (Zn) | d-block |
| f-orbital | 14 | Uranium (U), Cerium (Ce), Neodymium (Nd) | f-block |
⚙️ The Role of Orbitals in Chemical Properties
Orbitals explain why some elements are reactive while others are stable. For instance, noble gases have completely filled outer orbitals, making them chemically inert. In contrast, elements like sodium (Na) and chlorine (Cl) react readily because they seek to fill or empty their valence shells for stability.
🔬 Quantum Mechanics and the Periodic Table
The concept of orbitals stems from quantum mechanics, particularly Schrödinger’s wave equation. It describes the probability distribution of electrons, which gives rise to orbital shapes spherical (s), dumbbell-shaped (p), and complex (d and f). This quantum view connects directly to modern chemical theory.
💡 Summary
Orbitals shape the very identity of elements. Understanding how they are distributed on the periodic table allows chemists to predict chemical reactivity, bonding, and even the color of compounds. The table’s design isn’t just aesthetic it’s a blueprint of atomic structure and behavior.
Frequently Asked Questions (FAQs)
-
Aim for 4-6 focused hours, ensuring you incorporate breaks to avoid burnout.
-
Practice mindfulness techniques, take practice exams under realistic conditions, and maintain a balanced lifestyle.
-
Set short-term goals, seek support from mentors, and reward yourself for small achievements.
-
Regular exercise improves focus, reduces stress, and enhances overall mental clarity.
-
KOTC offers personalized learning tools, gamification features, and adaptive question banks to help students stay on track without burnout.