⚗️ Buffer Solutions: Definition & Function for the MCAT
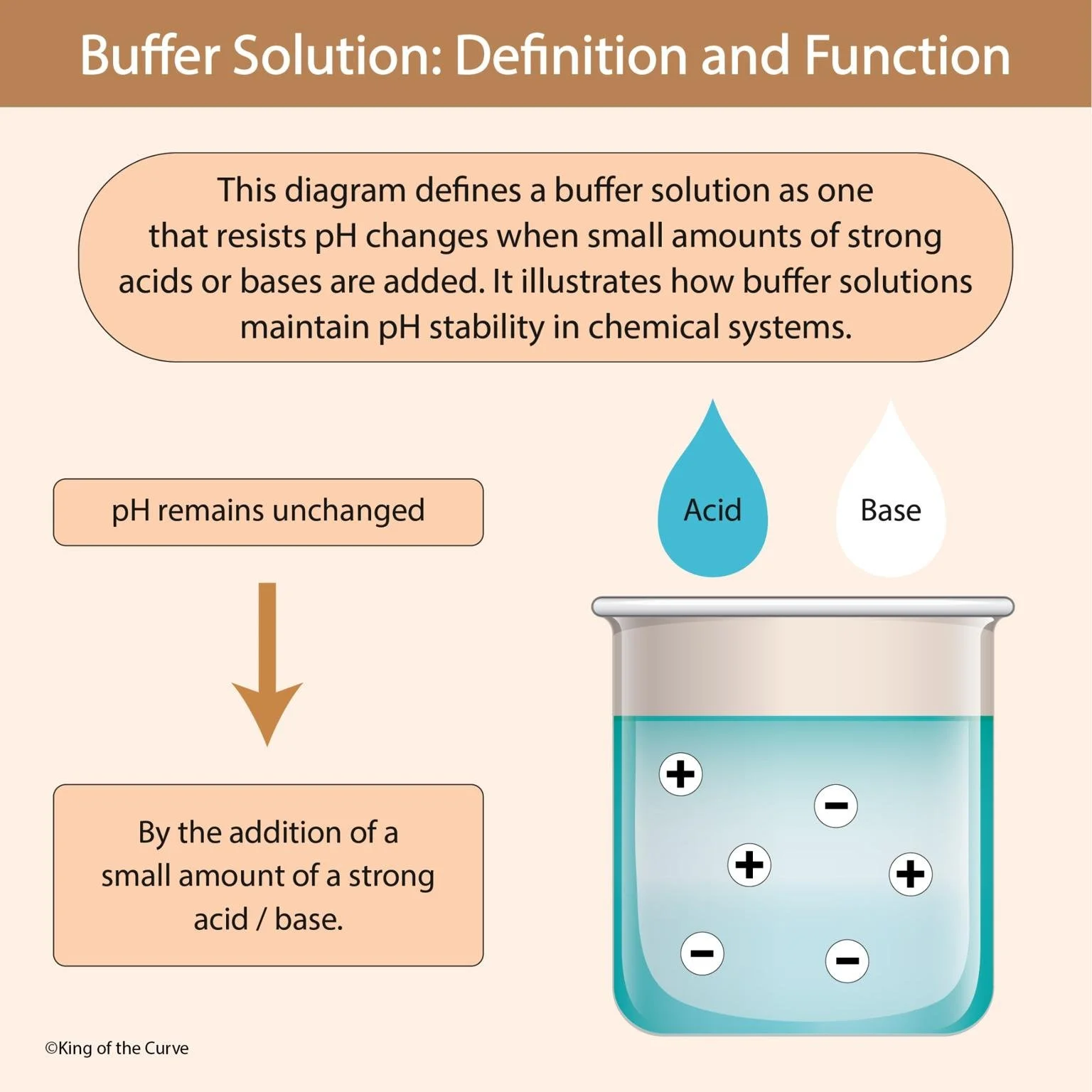
A buffer solution is a chemical system that resists changes in pH when small amounts of strong acid or base are added. This is essential in both biological systems (like blood) and laboratory experiments, where stable pH is crucial for proper chemical reactions and physiological processes. Without buffers, even tiny pH shifts could denature proteins, alter reaction rates, or disrupt normal bodily functions.
🧪 How Buffers Work
Buffers contain a weak acid and its conjugate base (or vice versa). These two components work together to neutralize added acids or bases:
When acid (H⁺) is added: The conjugate base binds with H⁺ ions, preventing a large increase in acidity.
When base (OH⁻) is added: The weak acid donates H⁺ ions to neutralize OH⁻, preventing a sharp rise in alkalinity.
Because of this balance, the pH remains nearly unchanged within the buffer’s effective range — usually close to the pKa of the acid in the buffer system.
🔍 MCAT & NCLEX Connection
MCAT: Buffers appear in acid-base chemistry, physiology, and biochemistry. You’ll often see them in blood pH regulation questions, such as the bicarbonate buffer system maintaining pH ~7.4.
NCLEX: Understanding buffer systems helps nurses interpret blood gas results and respond to acid-base imbalances like metabolic acidosis or alkalosis.
💡 Test Tip: The Henderson-Hasselbalch equation is your go-to formula:
It lets you calculate pH when you know the concentrations of the weak acid and conjugate base.
🌍 Real-Life Examples of Buffers
Blood plasma: The bicarbonate buffer keeps blood pH stable despite daily metabolic changes.
Biochemical reactions: Many enzymes require a narrow pH range to function optimally; buffers maintain this.
Food preservation: Buffers help control acidity in drinks and processed foods, extending shelf life.
Pharmaceuticals: Drug formulations often include buffers to maintain chemical stability in the body.
🧠 Why Buffers Matter Beyond the Exam
Buffers are not just a test topic — they’re a cornerstone of life sciences. They’re why your blood doesn’t swing from acidic to alkaline every time you eat, why your cells survive environmental changes, and why lab experiments can yield consistent results.
🚀 How KOTC Helps You Master Chemistry
With over 100,000 downloads, King of the Curve offers:
Adaptive Q-Bank for personalized practice in acid-base chemistry.
Daily questions to keep your skills sharp.
Gamified learning with Curve Coins to make studying rewarding.
Frequently Asked Questions (FAQs)
-
Aim for 4-6 focused hours, ensuring you incorporate breaks to avoid burnout.
-
Practice mindfulness techniques, take practice exams under realistic conditions, and maintain a balanced lifestyle.
-
Set short-term goals, seek support from mentors, and reward yourself for small achievements.
-
Regular exercise improves focus, reduces stress, and enhances overall mental clarity.
-
KOTC offers personalized learning tools, gamification features, and adaptive question banks to help students stay on track without burnout.