Henderson-Hasselbalch Equation Made Simple: Buffer Systems for USMLE Step 1
Some equations come and go. But the Henderson-Hasselbalch equation is one you’ll see everywhere—from blood gas analysis to renal physiology. If you're prepping for USMLE Step 1, mastering this equation helps you dominate topics like acid-base disorders, biochemistry, and buffer systems.
Today, we’re going to make this classic formula stick with an easy breakdown and a KOTC visual that ties it all together.
📘 What Is the Henderson-Hasselbalch Equation?
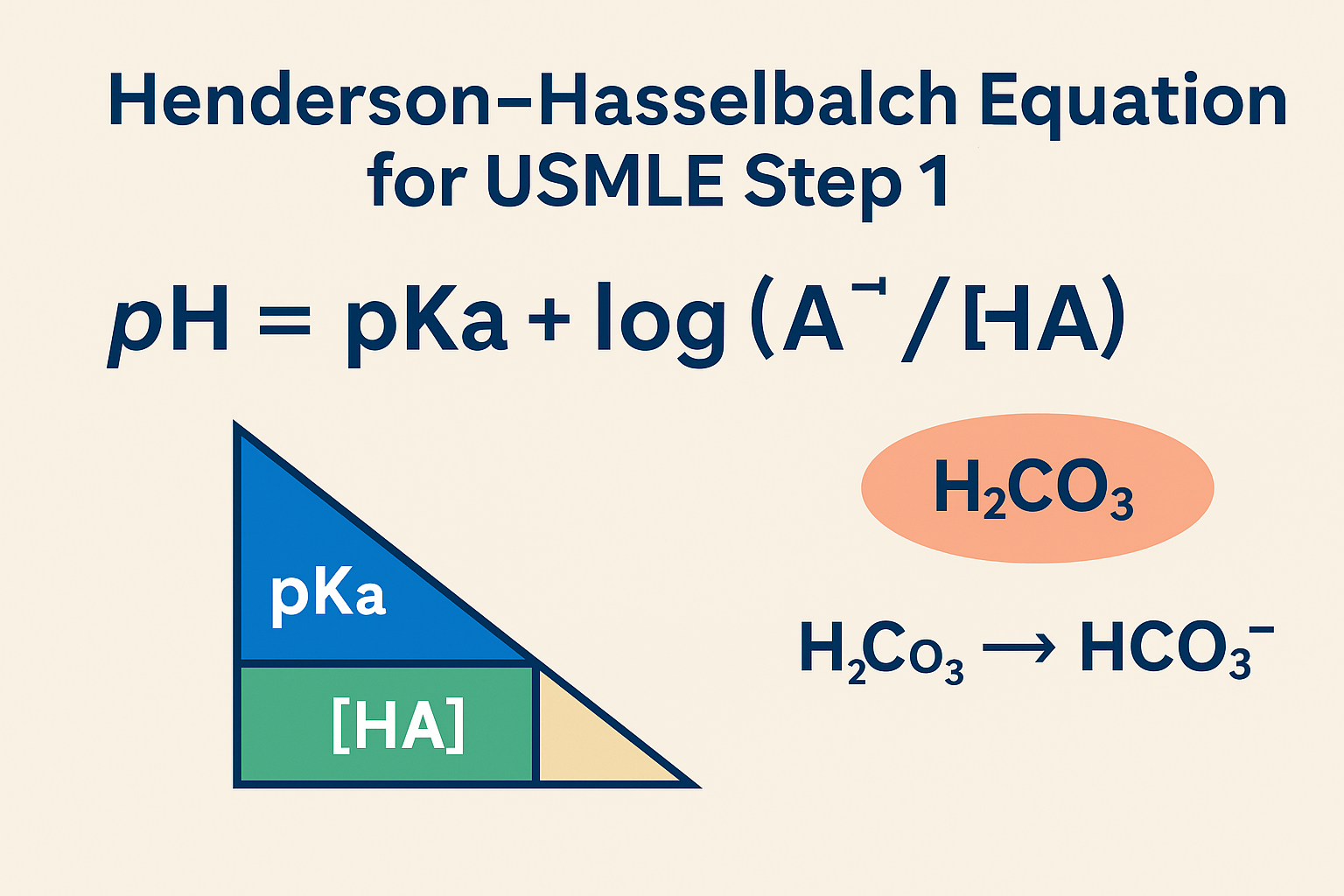
pH = pKa + log ([A⁻]/[HA])
Where:
pH = acidity of the solution
pKa = acid dissociation constant (a property of the buffer)
[A⁻] = concentration of the conjugate base
[HA] = concentration of the weak acid
💡 Why This Equation Matters for Step 1
Appears in Biochemistry, Physiology, and Pathology questions
Helps explain how buffers resist changes in pH
Is central to understanding respiratory and metabolic compensation
This formula underpins bicarbonate buffering in the blood (HCO₃⁻/H₂CO₃ system) and ties directly into acid-base vignettes, so you’ll see it in ABG interpretation questions too.
🧪 Buffer Example: Bicarbonate in the Blood
The primary buffer system in the body is:
H₂CO₃ ⇌ H⁺ + HCO₃⁻
🧠 When blood becomes too acidic, bicarbonate (HCO₃⁻) binds excess H⁺.
🫁 When it becomes too basic, carbonic acid (H₂CO₃) dissociates to release H⁺.
Using the Henderson-Hasselbalch formula:
pH = 6.1 + log ([HCO₃⁻]/0.03 × pCO₂)
This version lets you calculate blood pH from bicarbonate and CO₂ levels—perfect for clinical vignette math.
🧠 USMLE-Style Example Question
A 27-year-old man with Type 1 diabetes presents with fruity breath and rapid breathing. ABG shows:
pH 7.10, pCO₂ 20, HCO₃⁻ 10.What does the Henderson-Hasselbalch equation reveal?
Answer: This is metabolic acidosis with partial respiratory compensation. Use the formula to confirm the acidic shift.
🔁 Key Takeaways for Step 1
Know both the classic and bicarbonate-specific versions of the equation.
Understand how buffers resist pH changes, not neutralize them.
Practice using log rules:
log(1) = 0,log(10) = 1, andlog(0.1) = -1These come up more than you think!
🎓 Call-To-Action
Want more visuals like this one—plus 1000+ additional cheat sheets, formulas, and mapped-out concepts?
🎯 Try the KOTC Classroom for free:
🔗 https://kingofthecurve.org/trial-sessions
Or level up with lifetime access:
🔗 https://kingofthecurve.org/free-lifetime
Frequently Asked Questions (FAQs)
-
Aim for 4-6 focused hours, ensuring you incorporate breaks to avoid burnout.
-
Practice mindfulness techniques, take practice exams under realistic conditions, and maintain a balanced lifestyle.
-
Set short-term goals, seek support from mentors, and reward yourself for small achievements.
-
Regular exercise improves focus, reduces stress, and enhances overall mental clarity.
-
KOTC offers personalized learning tools, gamification features, and adaptive question banks to help students stay on track without burnout.