💧 DAT Solubility Rules & Precipitation Reactions: What Dissolves and What Doesn’t
You’ll often face DAT questions asking:
Whether a precipitate forms
How to write complete vs. net ionic equations
Which salts are soluble in water
These are quick-win questions—if you memorize the rules.
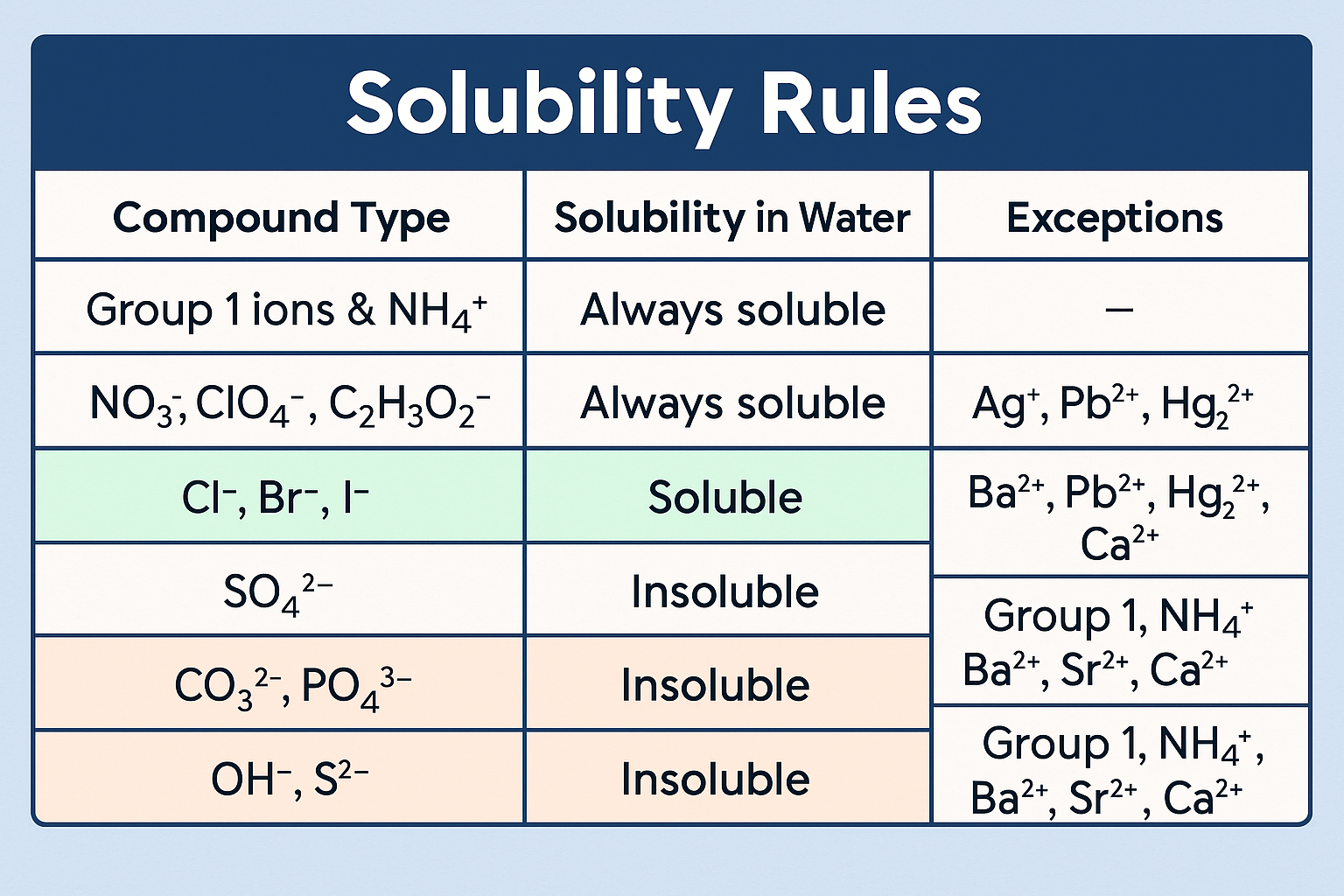
📋 Core Solubility Rules to Know
| Compound Type | Solubility in Water | Exceptions |
|---|---|---|
| Group 1 ions & NH₄⁺ | Always soluble | — |
| NO₃⁻, ClO₄⁻, C₂H₃O₂⁻ | Always soluble | Ag⁺, Pb²⁺, Hg₂²⁺ |
| Cl⁻, Br⁻, I⁻ | Soluble | Ba²⁺, Pb²⁺, Hg₂²⁺, Ca²⁺ |
| SO₄²⁻ | Insoluble | Group 1, NH₄⁺, Ba²⁺, Sr²⁺, Ca²⁺ |
| CO₃²⁻, PO₄³⁻ | Insoluble | Group 1, NH₄⁺ |
| OH⁻, S²⁻ | Insoluble | Group 1, NH₄⁺, Ba²⁺, Sr²⁺, Ca²⁺ |
🔎 How to Predict Precipitation on the DAT
Precipitation reactions occur when two soluble salts react to form an insoluble product (the precipitate). Use the solubility rules to identify which product is solid.
Example:
What forms when AgNO₃ is mixed with NaCl?
Answer:
Ag⁺ + Cl⁻ → AgCl (s) (precipitate)
Na⁺ + NO₃⁻ → NaNO₃ (aqueous)
⚖️ How to Write Net Ionic Equations
Write balanced molecular equation
Break apart soluble strong electrolytes
Cancel spectator ions
Write the net ionic reaction
Example:
Molecular:
Net Ionic:
🧠 DAT Tips for Solubility & Reactions
Group 1 and NH₄⁺ = always soluble
Ag⁺, Pb²⁺, and Hg₂²⁺ = often form precipitates
Use the (aq) vs (s) labels to determine reaction type
Watch for double displacement reactions and lab-style setups
🎯 Call to Action
Ready to master every DAT solubility rule visually and interactively?
👩🔬 Dive into King of the Curve’s Visual Vault, Curve Coin gamification, and multiplayer review sessions.
👉 Join the Free KOTC Platform Now
✅ Summary
Know solubility rules and key exceptions cold
Use them to quickly identify precipitate products
Practice net ionic equations regularly
Visual memory tools make this fast and permanent
Frequently Asked Questions (FAQs)
-
Aim for 4-6 focused hours, ensuring you incorporate breaks to avoid burnout.
-
Practice mindfulness techniques, take practice exams under realistic conditions, and maintain a balanced lifestyle.
-
Set short-term goals, seek support from mentors, and reward yourself for small achievements.
-
Regular exercise improves focus, reduces stress, and enhances overall mental clarity.
-
KOTC offers personalized learning tools, gamification features, and adaptive question banks to help students stay on track without burnout.