⚖️ Le Chatelier’s Principle Explained: DAT Chemistry Equilibrium Strategy
If you're prepping for the DAT General Chemistry section, expect to encounter equilibrium problems. Le Chatelier’s Principle is a favorite because it tests critical thinking, not just formula recall.
Whether it's a change in concentration, temperature, volume, or pressure, understanding how a system reacts can quickly score you points.
🔬 What Is Le Chatelier’s Principle?
Le Chatelier’s Principle states:
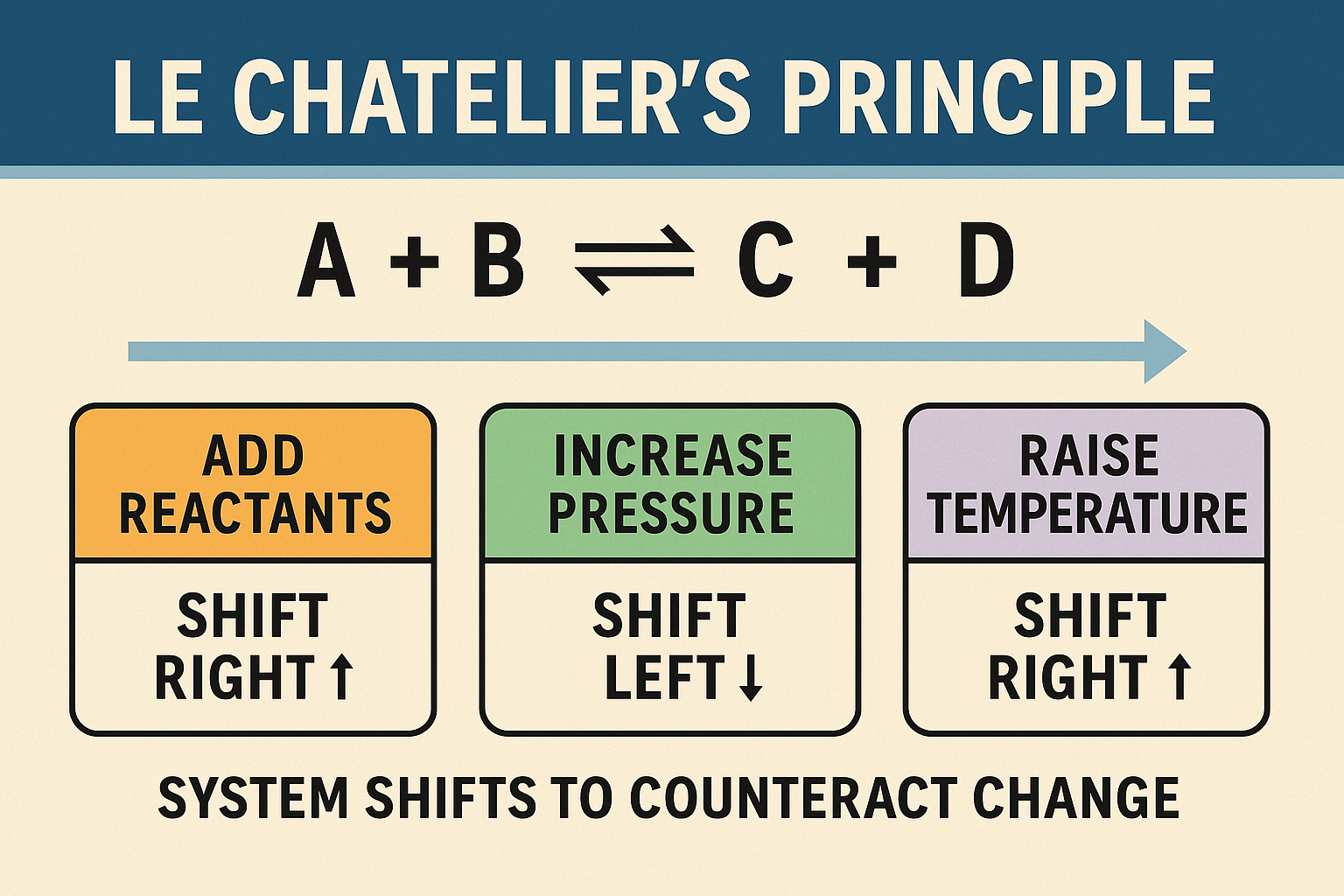
“If a dynamic equilibrium is disturbed by changing the conditions, the system responds to counteract the change and restore equilibrium.”
🔁 How Changes Affect Equilibrium
| Change Applied | System’s Response |
|---|---|
| Add Reactants | Shift → Products |
| Add Products | Shift → Reactants |
| Remove Reactants | Shift ← Reactants (replenish) |
| Increase Pressure | Shift → Side with fewer gas moles |
| Decrease Pressure | Shift → Side with more gas moles |
| Increase Temperature | Shift in endothermic direction |
| Decrease Temperature | Shift in exothermic direction |
🧠 DAT Question Format
You may see Le Chatelier’s Principle tested by:
Predicting which direction a reaction shifts
Determining how changes affect equilibrium concentration
Comparing shifts in exothermic vs. endothermic reactions
Interpreting graphical equilibrium data
🌡️ Endothermic vs. Exothermic Shifts
| Type of Reaction | Temp ↑ Causes Shift Toward | ΔH Sign |
|---|---|---|
| Endothermic | Products | + |
| Exothermic | Reactants | – |
Key DAT Tip: Treat heat as a "reactant" or "product" based on reaction type.
💬 Example DAT-Style Question
In the following reaction:
What happens if temperature increases?
✅ Answer: The reaction shifts left (heat added → equilibrium shifts to absorb it).
🎯 Call to Action
Want to drill more DAT-style scenarios like this? Our Adaptive Q-Bank and Visual Vault let you master equilibrium questions with instant feedback.
👉 Join King of the Curve for Free
🚀 Boost your DAT score using Curve Coins, real-time multiplayer, and spaced repetition tools.
✅ Summary
Le Chatelier’s Principle explains how systems at equilibrium respond to external changes.
Key triggers: concentration, pressure, temperature.
Learn to predict shifts and apply them to reaction dynamics.
Frequently Asked Questions (FAQs)
-
Aim for 4-6 focused hours, ensuring you incorporate breaks to avoid burnout.
-
Practice mindfulness techniques, take practice exams under realistic conditions, and maintain a balanced lifestyle.
-
Set short-term goals, seek support from mentors, and reward yourself for small achievements.
-
Regular exercise improves focus, reduces stress, and enhances overall mental clarity.
-
KOTC offers personalized learning tools, gamification features, and adaptive question banks to help students stay on track without burnout.