🧪 DAT Daily Blog: Colligative Properties Explained — DAT Chemistry Essentials
Colligative properties depend only on the number of solute particles, not their identity.
They commonly appear in DAT chemistry questions that involve conceptual logic and formula calculations.
This blog covers the four key properties—explained with visuals and tips to remember them.
🧊 What Are Colligative Properties?
Colligative properties are physical changes to solvents caused by the addition of non-volatile solutes.
They depend on the concentration of particles, not the type of solute.
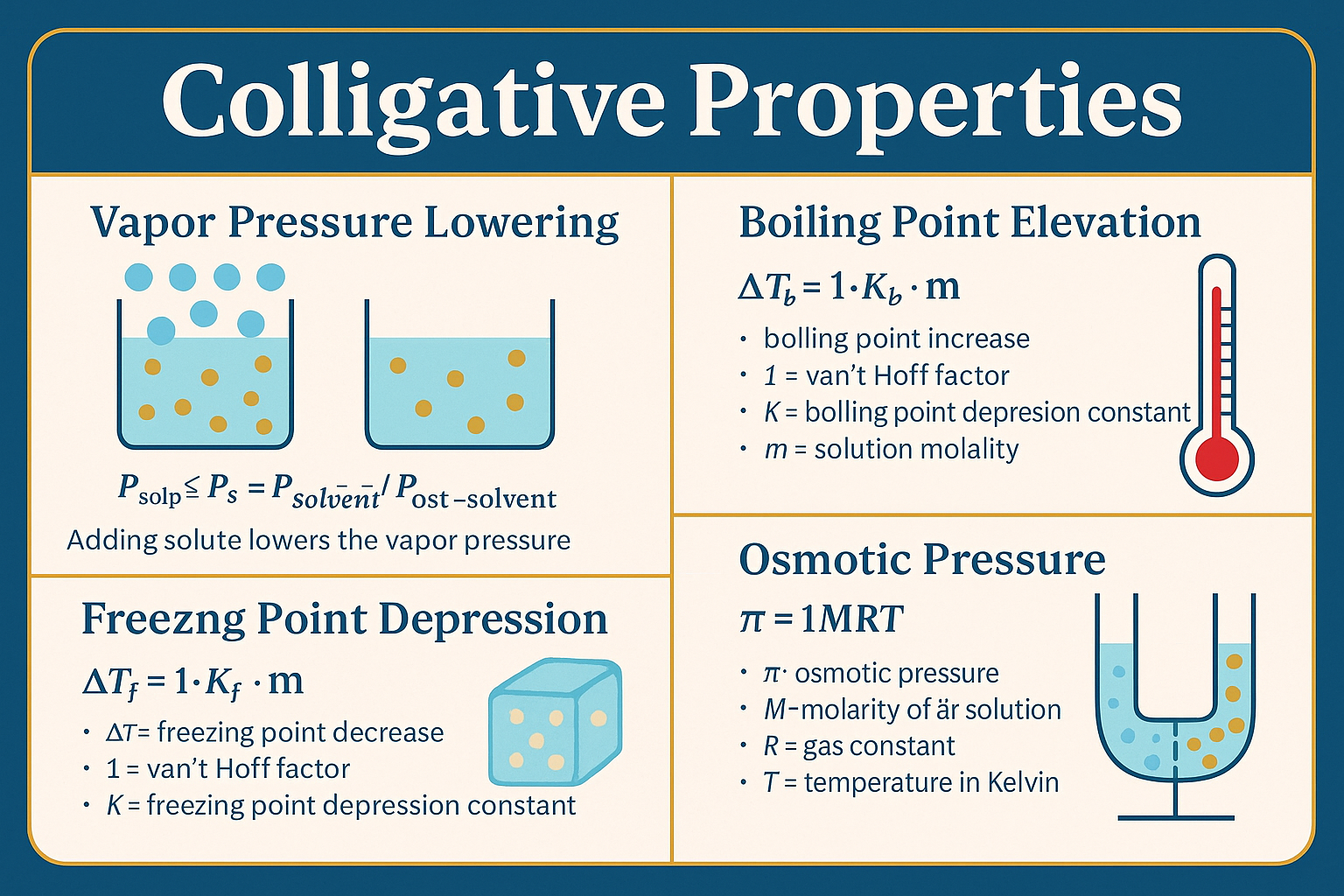
4 Key Colligative Properties on the DAT:
| Property | Effect |
|---|---|
| Vapor Pressure Lowering | Decreases vapor pressure |
| Boiling Point Elevation | Increases boiling point |
| Freezing Point Depression | Lowers freezing point |
| Osmotic Pressure | Increases water influx/osmosis |
🔥 Boiling Point Elevation
- ΔTb: Increase in boiling point
- i: van’t Hoff factor (number of solute particles)
- Kb: Boiling point elevation constant
- m: Molality of the solution
📌 DAT Tip: More particles = higher boiling point.
❄️ Freezing Point Depression
- ΔTf: Decrease in freezing point
- i: van’t Hoff factor (number of solute particles)
- Kf: Freezing point depression constant
- m: Molality of the solution
🔍 Example: Adding salt to icy roads lowers the freezing point, preventing refreezing.
💨 Vapor Pressure Lowering (Raoult’s Law)
- Xsolvent: Mole fraction of the solvent
- P0solvent: Vapor pressure of the pure solvent
- Effect: Adding a non-volatile solute lowers the vapor pressure
💧 Osmotic Pressure
- π: Osmotic pressure
- i: van’t Hoff factor (number of particles)
- M: Molarity of the solution
- R: Ideal gas constant
- T: Temperature in Kelvin
📌 Important for understanding water movement across membranes (osmotic balance in biology).
🎓 Quick Reference Table
| Concept | Formula | Triggered By |
|---|---|---|
| Boiling Point Elev. | ΔTb = i · Kb · m | More solute |
| Freezing Pt. Depr. | ΔTf = i · Kf · m | More solute |
| Vapor Pressure | P = Xsolvent · P°solvent | Mole fraction |
| Osmotic Pressure | π = i · M · R · T | Water flow |
🧠 DAT Question Styles
Calculation: “How much will the boiling point increase with 0.2 m NaCl?”
Conceptual: “Why does a sugar solution freeze at a lower temperature?”
Biology Crossover: “Which property explains water entering a hypertonic cell?”
🎯 CTA: Master DAT Chemistry with KOTC
Get access to:
Visual-rich chemistry decks
Practice problems on all colligative property types
DAT-specific study tools & daily questions
Frequently Asked Questions (FAQs)
-
Aim for 4-6 focused hours, ensuring you incorporate breaks to avoid burnout.
-
Practice mindfulness techniques, take practice exams under realistic conditions, and maintain a balanced lifestyle.
-
Set short-term goals, seek support from mentors, and reward yourself for small achievements.
-
Regular exercise improves focus, reduces stress, and enhances overall mental clarity.
-
KOTC offers personalized learning tools, gamification features, and adaptive question banks to help students stay on track without burnout.