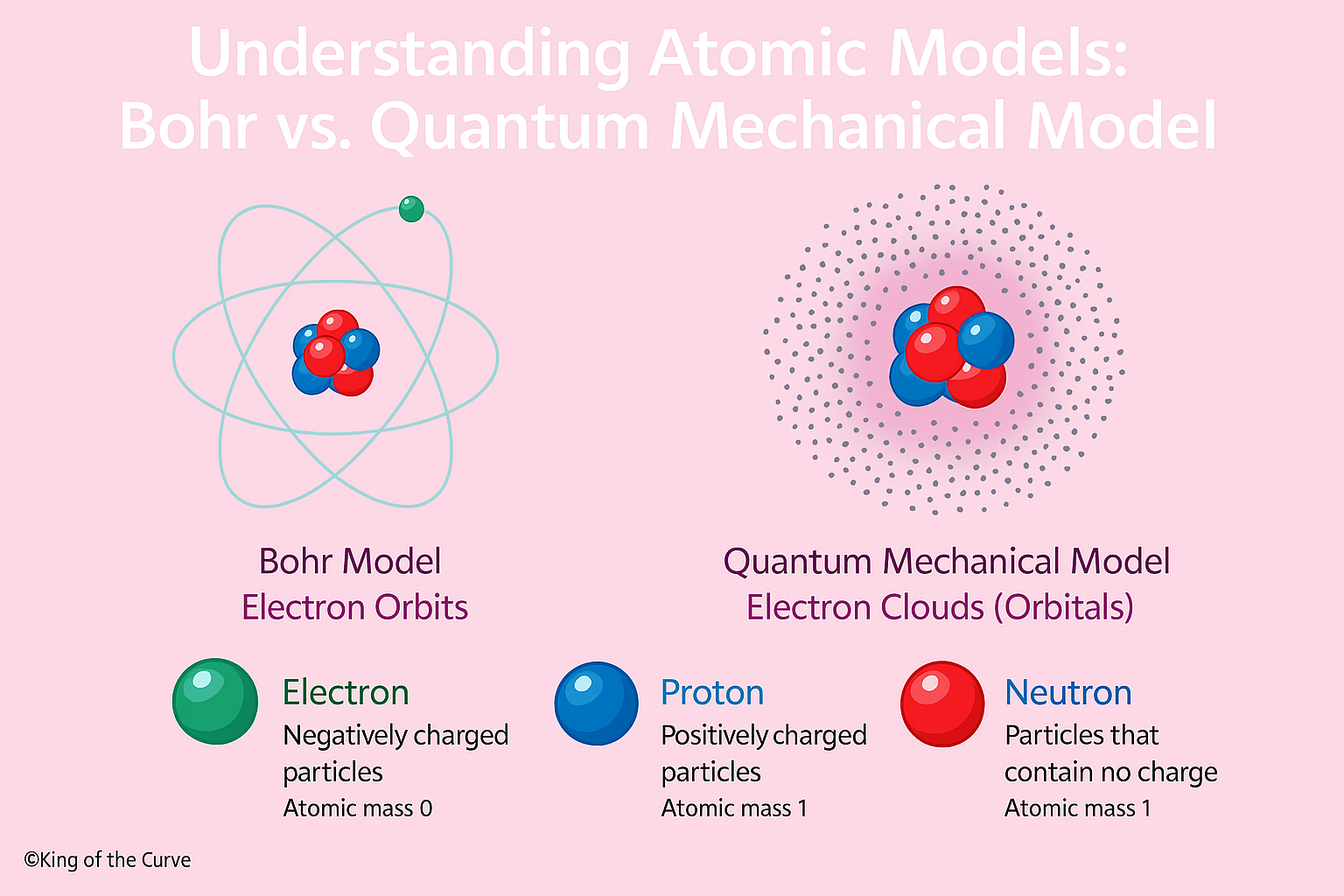
🧠 Understanding Atomic Models: Bohr vs. Quantum Mechanical Model
Every aspiring medical student must wrestle with foundational chemistry — and one of the most crucial stepping stones is understanding how atoms are structured. Today, I’m going to show you the difference between two pivotal atomic models: the Bohr Model and the Quantum Mechanical Model.
These concepts don’t just show up in textbooks — they are embedded in MCAT chemistry passages and foundational to understanding electron behavior in medicine and biology. Let’s break them down using a proprietary King of the Curve (KOTC) science visual to help cement your learning.
🔬 Bohr Model: Electrons in Defined Orbits
The Bohr Model, developed by Niels Bohr in the early 20th century, revolutionized our understanding of atomic structure. In this model:
Electrons travel in fixed circular orbits around the nucleus.
Each orbit corresponds to a discrete energy level.
Energy is absorbed or released when electrons jump between orbits.
This model was critical in explaining atomic emission spectra, especially for hydrogen.
MCAT Tip: You'll often be asked to interpret spectral lines or energy transitions in physics and chemistry passages. Remember that each orbital jump involves quantized energy — a perfect place to apply the Bohr model.
🧪 Quantum Mechanical Model: Electron Clouds
Modern chemistry takes things a step further. The Quantum Mechanical Model accounts for:
Electron probability distributions, not fixed paths.
Electrons exist in regions called orbitals where they are likely to be found.
This model embraces the Heisenberg Uncertainty Principle — we can’t know an electron’s exact position and momentum simultaneously.
MCAT Tip: Expect questions about s, p, d, and f orbitals and how they relate to periodic trends, bonding, and molecular geometry. Mastering this model is key to tackling organic chemistry questions, too.
⚛️ Subatomic Particles Cheat Sheet
| Particle | Charge | Atomic Mass |
|---|---|---|
| Electron | Negative | 0 |
| Proton | Positive | 1 |
| Neutron | None | 1 |
🔁 Quick Recap
| Feature | Bohr Model | Quantum Mechanical Model |
|---|---|---|
| Electron Path | Fixed circular orbits | Probabilistic electron clouds |
| Key Concept | Quantized energy levels | Orbitals with uncertainty |
| Useful For | Spectroscopy, hydrogen atom | Modern chemistry, bonding, MCAT |
✅ Final Thoughts + Call-to-Action
Mastering atomic models is more than memorizing diagrams — it's about applying theory to practice, especially in high-stakes exams like the MCAT. Let KOTC’s engaging visuals and adaptive tools be your study sidekick.
💡 Explore our full learning platform: kingofthecurve.org/pre-med-essentialsre of yourself, because smart, rested students make the best doctors.
Frequently Asked Questions (FAQs)
-
Aim for 4-6 focused hours, ensuring you incorporate breaks to avoid burnout.
-
Practice mindfulness techniques, take practice exams under realistic conditions, and maintain a balanced lifestyle.
-
Set short-term goals, seek support from mentors, and reward yourself for small achievements.
-
Regular exercise improves focus, reduces stress, and enhances overall mental clarity.
-
KOTC offers personalized learning tools, gamification features, and adaptive question banks to help students stay on track without burnout.